
호환 APK 다운로드
| 다운로드 | 개발자 | 평점 | 리뷰 |
|---|---|---|---|
|
Stoichiometric equation
다운로드 Apk Playstore 다운로드 → |
Flutter App Development |
3 | 100 |
|
Stoichiometric equation
다운로드 APK |
Flutter App Development |
3 | 100 |
|
Stoichiometry Plus
다운로드 APK |
thermobook.net | 2.9 | 4 |
|
Stoichiometry
다운로드 APK |
Wiki Kids Limited | 3 | 100 |
|
Stoichiometry Pro
다운로드 APK |
thermobook.net | 3 | 100 |
|
Chemical Equation Balancer 다운로드 APK |
KharbLabs | 3.6 | 7 |
|
Epic: Kids' Books & Reading 다운로드 APK |
Epic Kids Inc. | 4.1 | 25,243 |
다른 한편에서는 원활한 경험을하려면 파일을 장치에 다운로드 한 후 파일을 사용하는 방법을 알아야합니다. APK 파일은 Android 앱의 원시 파일이며 Android 패키지 키트를 의미합니다. 모바일 앱 배포 및 설치를 위해 Android 운영 체제에서 사용하는 패키지 파일 형식입니다.
네 가지 간단한 단계에서 사용 방법을 알려 드리겠습니다. Stoichiometry 귀하의 전화 번호.
아래의 다운로드 미러를 사용하여 지금 당장이 작업을 수행 할 수 있습니다. 그것의 99 % 보장 . 컴퓨터에서 파일을 다운로드하는 경우, 그것을 안드로이드 장치로 옮기십시오.
설치하려면 Stoichiometry 타사 응용 프로그램이 현재 설치 소스로 활성화되어 있는지 확인해야합니다. 메뉴 > 설정 > 보안> 으로 이동하여 알 수없는 소스 를 선택하여 휴대 전화가 Google Play 스토어 이외의 소스에서 앱을 설치하도록 허용하십시오.
이제 위치를 찾으십시오 Stoichiometry 방금 다운로드 한 파일입니다.
일단 당신이 Stoichiometry 파일을 클릭하면 일반 설치 프로세스가 시작됩니다. 메시지가 나타나면 "예" 를 누르십시오. 그러나 화면의 모든 메시지를 읽으십시오.
Stoichiometry 이 (가) 귀하의 기기에 설치되었습니다. 즐겨!
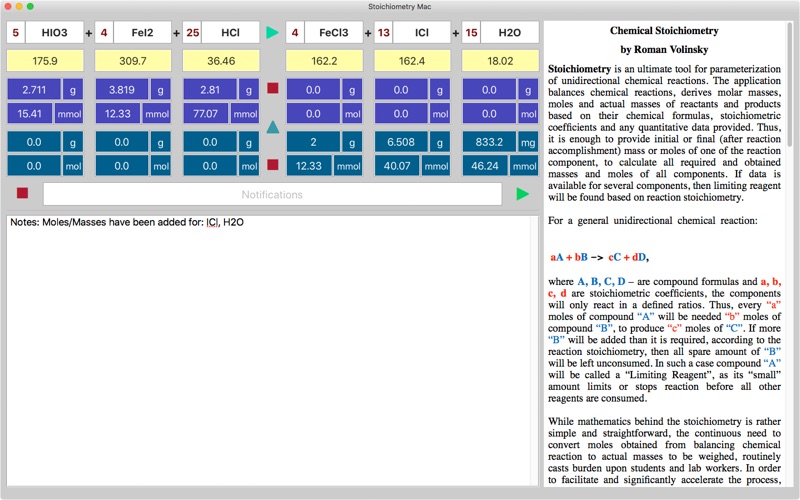
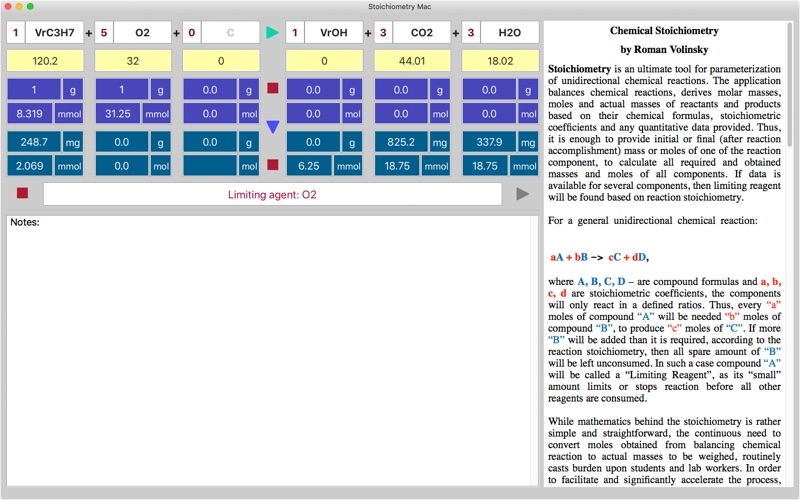
Stoichiometry for Mac is an ultimate tool for balancing chemical reactions, finding molar masses, moles and actual masses of reactants and products based on their chemical formulas, stoichiometric coefficients and any quantitative data provided. It is enough to provide initial or final mass or moles of one of the reaction component, to calculate all required and obtained masses and moles of all components. Limiting reagent will be found based on the reaction stoichiometry. For chemical reaction: aA + bB -> cC + dD, the components will only react in a defined ratios. Thus, every “a” moles of compound “A” will need “b” moles of compound “B”, to produce “c” moles of “C”. If more “B” will be added than it is required, then all spare amount of “B” will be left unconsumed. In such a case compound “A” will be called a “Limiting Reagent”, as its “small” amount limits or stops reaction before all other reagents are consumed. While math behind the stoichiometry is rather simple and straightforward, the continuous need to convert moles obtained from balancing chemical reaction to actual masses to be weighed, routinely casts burden upon students and lab workers. In order to facilitate and significantly accelerate the process, the Stoichiometry application has been developed. Let’s consider the working example: Example 1: How many moles of HCl are needed in order to obtain 2.0 g of FeCl3, in the reaction: HCl + FeI2 + HCl -> FeCl3 + ICl + H20 The first step is balancing chemical reaction and finding molar masses, since moles-mass conversion is needed. All is done automatically by pressing the orange arrow connecting reagents and products (the screenshot is provided): 5HClO3 + 4FeI2 + 25HCl -> 4FeCl3 + 13ICl + 15H20, The next step is to find the moles of FeCl3 from the mass (2.0 g), and then convert them to moles of HCl based on 4:25 ratio obtained from the balanced reaction. Stoichiometry application provides data table that comprised of masses and moles rows for data available before reaction initiated (coloured blue), and data rows for component amounts that obtained or left after the reaction is accomplished (coloured violet). When the direction of calculation arrow, that is located between the initial and final data rows, is turned downwards the final masses and moles are calculated from the initially provided amounts of reactant and products, and when it turned upwards, reactants and products amounts that would be required to achieve the provided final state are calculated. The later is exactly matches our situation, as we need to find the initial amount of HCl from the final, known FeCl3 mass. So we press the arrow to turn it upwards, set the FeCl3 mass to the final mass field and press calculate button to automatically fill the table with all masses and moles for all components. Apparently, 77.07 mmoles of HCl are required to get 2.0 g of FeCl. Example 2: Using the same reaction: 5HClO3 + 4FeI2 + 25HCl -> 4FeCl3 + 13ICl + 15H20, Find out the limiting reagent, if 3.0 grams of each reactant have been mixed initially. Solution using Stoichiometry application: The direction arrow should be turned downwards, since initial data is provided for calculations. Next, masses should be set for all reagents in blue mass fields and then pressing the calculate button populates the final violet cells for reactants that left and products that are obtained. The application alerts on FeI2 as a limiting reagent, and indeed all 9.687 mmoles of the FeI2 are consumed, while other reagents are partially left unused. Important points: Charge, if exist must be provided as well, separated by coma: Na,+ . Application supports state indexes: (g), (s), (l), (aq), lowercased only, placed at the end of formula: OH,-(aq), CO2(g), are considered to be a crucial part of a molecule, so same molecules of different states appearing on the opposite sides of equation are not cancelled!